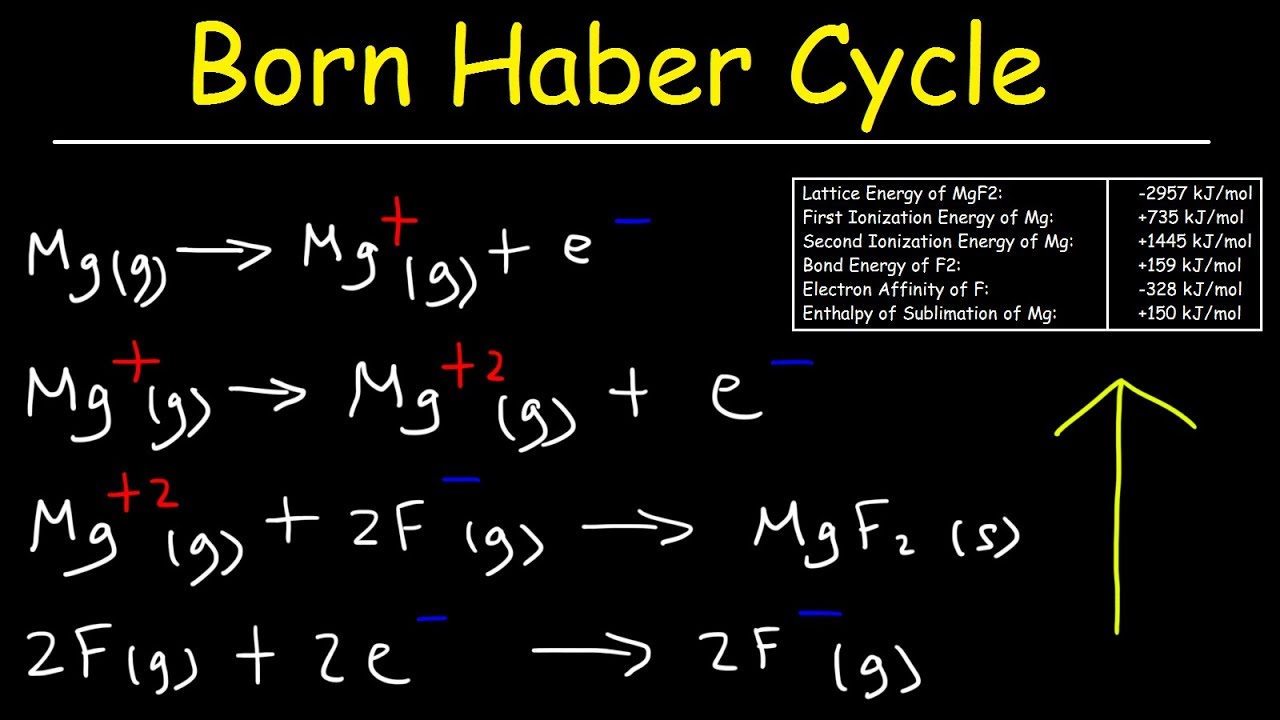
 This chemistry provides a basic introduction into the born haber cycle. It explains how to calculate the enthalpy of formation of a compound using the lattice energy, first ionization energy and second ionization energy values, electron affinity, and bonding energy for the substances that make up the compound. It explains how to use hess law to calculate the enthalpy of combustion as well as the enthalpy of formation of certain substances. This video contains 1 practice problem / example.
This chemistry provides a basic introduction into the born haber cycle. It explains how to calculate the enthalpy of formation of a compound using the lattice energy, first ionization energy and second ionization energy values, electron affinity, and bonding energy for the substances that make up the compound. It explains how to use hess law to calculate the enthalpy of combustion as well as the enthalpy of formation of certain substances. This video contains 1 practice problem / example.
New Chemistry Video Playlist:
https://www.youtube.com/watch?v=bka20...
Access to Premium Videos:
https://www.patreon.com/MathScienceTutor
Facebook: https://www.facebook.com/MathScienceT...
Born Haber Cycle, Basic Introduction, Lattice Energy, Hess Law & Enthalpy of Formation - Chemistry chemistry jobs | |
| 1,099 Likes | 1,099 Dislikes |
| 134,251 views views | 1.16M followers |
| Education | Upload TimePublished on 23 Oct 2017 |
Không có nhận xét nào:
Đăng nhận xét